Why Safetymint Permit to Work System for Pharmaceuticals
Built for the regulated world of pharma, Safetymint helps you manage permits with precision, ensure compliance, and minimize disruption to validated environments.
Minimize Risk to Cleanrooms and Critical Areas
Control maintenance, calibration, and engineering tasks with clear approvals and checklists to avoid GMP violations or cross-contamination.
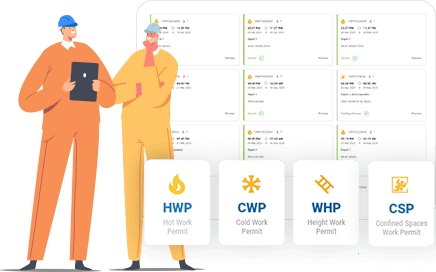
Support GMP and Quality Compliance
Document every permit action, approval, and closure to support audits and meet global pharma safety standards.
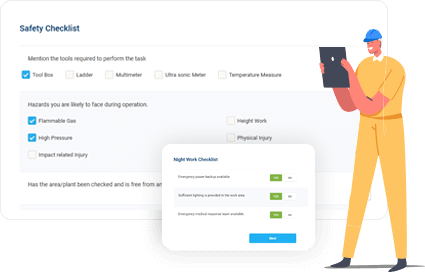
Improve Safety Without Slowing Production
Enable field and facility teams to manage permits quickly and safely—keeping operations compliant and on schedule.

Trusted by leading companies across industries:
Take a free trialKey Features
Why Choose Safetymint PTW System

Feature-rich and scalable
Built to meet the demands of multi-site pharmaceutical operations.

Highly customizable
Tailor workflows, permit types, and approvals to your quality and validation processes.

Multi-lingual support
Support multilingual teams across global pharma manufacturing sites.
Free permit templates
Accelerate adoption with industry-aligned templates for pharma use cases.
Dynamic checklist builder
Ensure site- and task-specific safety protocols are always followed.

Web and mobile access
Stay connected with permit workflows from any device, anywhere.

Customizable workflows
Align your PTW process with GMP and internal SOPs.

Real-time alerts and notifications
Keep everyone in the loop at every stage of permit execution.

E-signature support
Secure digital approvals that meet validation requirements.

Map plotting of work locations
Mark permit zones within your plant layout for better oversight.

Third-party integration
Connect with QMS, BMS, or maintenance platforms already in use.
Key Stakeholders & How Safetymint Helps
Safety Officers
Maintain oversight and ensure that all tasks follow safety and GMP protocols.


Facility Engineers
Raise, manage, and close work permits efficiently while maintaining documentation integrity.


Quality & Compliance Teams
Ensure all permit records support audits and align with regulatory standards.

Contractors & Vendors
Guide third-party staff through compliant permit workflows with clear approvals and responsibilities.
Elevate Your PTW Standards with Safetymint
Experience how Safetymint can simplify your Permit to Work management and improve compliance standards across your organization.